Introduction: Acute myeloid leukemia (AML) is a lethal hematopoietic malignancy, characterized by the accumulation of clonal myeloid progenitor cells arrested in development. AML patients have dismal outcomes with a 5-year overall survival (2011 - 2017) of 29.5% (SEER). The persistence of leukemia stem cells (LSCs), a treatment-resistant primitive cell population able to inititiate and maintain AML, is thought to underlie relapse. Even newly approved drugs do not target LSCs efficiently. MicroRNA (miR)-126 is a non-coding regulatory RNA that contributes to LSC homeostasis in the bone marrow niche. Several lines of evidence validate miR-126 as a target in AML. Thus, we designed and made a novel anti-miR-126 oligonucleotide (oligo) linked to a CpG moiety, termed miRisten. miRisten incorporates modifications such as 2'-fluoro and phosphorothioate substituting phosphodiester in the sugar moiety of the nucleotide to enhance in vivo stability. miRisten eradicates LSCs in mouse models of AML and synergizes with FDA-approved AML drugs (PMID: 29505034, 34372909). Here, the results of IND-enabling toxicokinetic (TK) studies of miRisten in rats and NHP are presented.
Objective: To investigate the pharmacological properties of miRisten to establish a safe dosing strategy to achieve prolonged knockdown of miR-126 for a first-in-human trial.
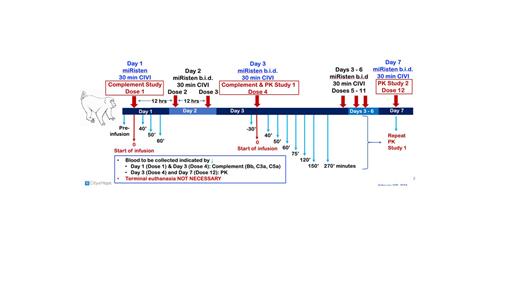
Experimental Plan and Results: Good laboratory practice (GLP) TK studies were carried out in Sprague-Dawley rats. miRisten was given IV b.i.d. for 19 days at human equivalent doses (HED) of 1.6 and 4 mg/kg. miRisten was measured in plasma after the first dose. Plasma miRisten C max average was 3622 ± 335 and 6010 ± 175 nM post first doses of 1.6 and 4mg/kg, respectively. Plasma t 1/2 averaged 20.0 ± 3.8 minutes across both doses. The area under the curve (AUC 0 - ¥) were 638.9 ± 32 nMxhr (1.6 mg/kg) and 1313.5 ± 439 (4 mg/kg). A parallel toxicology study was undertaken documenting cage-side observations and body weight; complement activation, blood chemistries, urine and hematology were also evaluated. There were no unscheduled deaths in the toxicology segment. Four single NHP exploratory studies were also carried out (Fig. 1: Treatment schema). In each study, miRisten was given IV as a slow bolus (~5') b.i.d. and PK parameters evaluated in plasma. Study #1 & #2: 1 wk at 4mg/kg; #3: 1 wk at 5 mg/kg; #4: 18 days at 4 mg/kg. In all studies, miRisten dosage was ramped up (1, 2, 4 mg/kg for all and 5 mg/kg at dose 4 for Study #3). Solumedrol was given as premedication for the first 3 doses in all studies to prevent known phosphorothioate-mediated complement activation and cytokine release syndrome. Blood draws for PK were carried out immediately following doses 3 and 12 (Studies 1 - 3) and after the last dose on Day 18 (Study 4). The average area under the curve AUC was 3860 ± 1189 (4 mg/kg) and 3954 ± 1569 nMxhr (5 mg/kg) which was greater than observed in mice given the equivalent HED in bolus doses (~6x, 465.0). The t 1/2 of plasma miRisten was 11.7 min on Day 3 and 12.0 min on Day 7, comparable to mouse (9.6 min) and rat (20 min). Cage-side observations, body weight, complement activation, blood chemistries, urine and hematology were also evaluated. There was no terminal sacrifice of NHP however, all animals tolerated the treatment without observable adverse events. Conclusion: The dose ramp-up in NHP combined with pre-medication with solumedrol were undertaken to prevent possible acute inflammatory reactivity leading potentially to death. There were no unscheduled deaths in any species studied. These data are consistent with the safety of miRisten given IV with a dose ramping regimen combined with solumedrol. The greater AUC in NHP may predict better potency in humans. In mice, miRisten at 1.6 mg/kg HED q.d. effectively eradicated LSCs [PMID 29505034].
Disclosures
Nguyen:Ostentus Therapeutics: Current equity holder in private company. Marcucci:Ostentus Therapeutics: Current equity holder in private company, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal